Page 32 - NAMAH-Jan-2018
P. 32
Namah Vol. 26, Issue 4, 15th January 2019
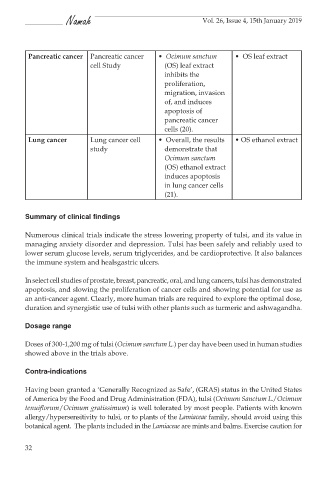
Pancreatic cancer Pancreatic cancer • Ocimum sanctum • OS leaf extract
cell Study (OS) leaf extract
inhibits the
proliferation,
migration, invasion
of, and induces
apoptosis of
pancreatic cancer
cells (20).
Lung cancer Lung cancer cell • Overall, the results • OS ethanol extract
study demonstrate that
Ocimum sanctum
(OS) ethanol extract
induces apoptosis
in lung cancer cells
(21).
Summary of clinical findings
Numerous clinical trials indicate the stress lowering property of tulsi, and its value in
managing anxiety disorder and depression. Tulsi has been safely and reliably used to
lower serum glucose levels, serum triglycerides, and be cardioprotective. It also balances
the immune system and healsgastric ulcers.
In select cell studies of prostate, breast, pancreatic, oral, and lung cancers, tulsi has demonstrated
apoptosis, and slowing the proliferation of cancer cells and showing potential for use as
an anti-cancer agent. Clearly, more human trials are required to explore the optimal dose,
duration and synergistic use of tulsi with other plants such as turmeric and ashwagandha.
Dosage range
Doses of 300-1,200 mg of tulsi (Ocimum sanctum L.) per day have been used in human studies
showed above in the trials above.
Contra-indications
Having been granted a ‘Generally Recognized as Safe’, (GRAS) status in the United States
of America by the Food and Drug Administration (FDA), tulsi (Ocimum Sanctum L./ Ocimum
tenuiflorum/Ocimum gratissimum) is well tolerated by most people. Patients with known
allergy/hypersensitivity to tulsi, or to plants of the Lamiaceae family, should avoid using this
botanical agent. The plants included in the Lamiaceae are mints and balms. Exercise caution for
32