Page 30 - NAMAH-Jan-2018
P. 30
Namah Vol. 26, Issue 4, 15th January 2019
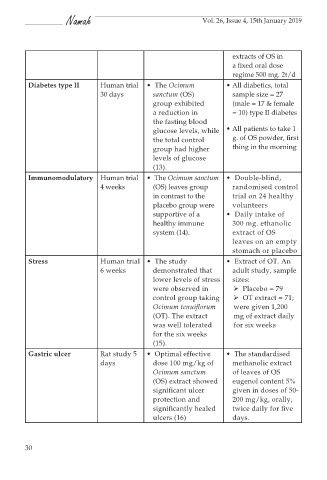
extracts of OS in
a fixed oral dose
regime 500 mg. 2t/d
Diabetes type II Human trial • The Ocimum • All diabetics, total
30 days sanctum (OS) sample size = 27
group exhibited (male = 17 & female
a reduction in = 10) type II diabetes
the fasting blood
glucose levels, while • All patients to take 1
the total control g. of OS powder, first
group had higher thing in the morning
levels of glucose
(13).
Immunomodulatory Human trial • The Ocimum sanctum • Double-blind,
4 weeks (OS) leaves group randomised control
in contrast to the trial on 24 healthy
placebo group were volunteers
supportive of a • Daily intake of
healthy immune 300 mg. ethanolic
system (14). extract of OS
leaves on an empty
stomach or placebo
Stress Human trial • The study • Extract of OT. An
6 weeks demonstrated that adult study, sample
lower levels of stress sizes:
were observed in Placebo = 79
control group taking OT extract = 71;
Ocimum tenuiflorum were given 1,200
(OT). The extract mg of extract daily
was well tolerated for six weeks
for the six weeks
(15).
Gastric ulcer Rat study 5 • Optimal effective • The standardised
days dose 100 mg/kg of methanolic extract
Ocimum sanctum of leaves of OS
(OS) extract showed eugenol content 5%
significant ulcer given in doses of 50-
protection and 200 mg/kg, orally,
significantly healed twice daily for five
ulcers (16) days.
30